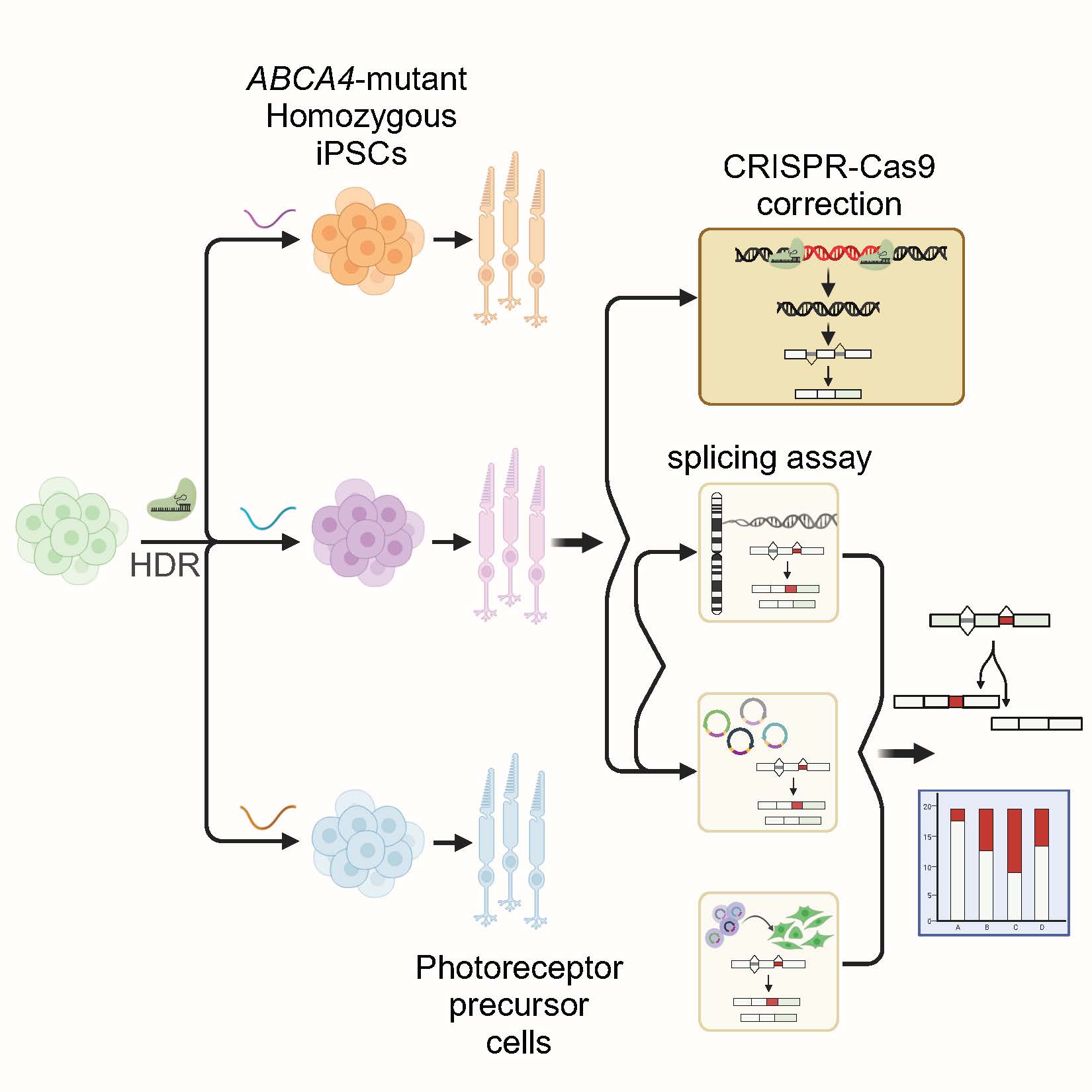
Identification of an efficient gene editing strategy for therapeutic applications.
In this study, the researchers combined and investigated the use of a minigene assay and isogenic PPCs, as superior qualitative and more accessible cellular models for the assessment and correction of splicing defects.
Certain recurrent point mutations in ABCA4 result in missplicing causing autosomal recessively inherited retinal disease (i.e., Stargardt disease, cone and cone-rod dystrohphy, retinitis pigmentosa) resulting in severe visual impairment.
In our relentless pursuit of functionally assessing and correcting splicing defects, with the aim of curing these blinding diseases, we have recently published our latest results in Molecular Therapy: Nucleic Acids with the following title "Splicing defects and CRISPR-Cas9 correction in isogenic homozygous photoreceptor precursors harboring clustered deep-intronic ABCA4 variants."
The research integrated the simplicity of minigene assays to test such splicing variants with state-of-the-art iPSC-differentiated photoreceptor precursor cells providing a more accessible cellular model to do so.
Given the rarity of such mutations, identifying patients with pathogenic splicing variants in a homozygous state is often challenging, while it would be the most suitable model to work with. Traditional functional studies in patient cell lines therefore often rely on heterozygous configurations, introducing a bias in the assessment of efficacy due to the presence of a different second mutation on the counter allele. To overcome this limitation, the involved researchers generated isogenic cell lines harboring the mutations of interest in homozygous state by CRISPR/Cas9-mediated knock-in. Subsequent differentiation into photoreceptor precursor cells enabled a precise assessment of the severity of the splicing defects in a retina-like context and proved minigene-transfected photoreceptor precursor cells to closely mimic the splicing defects endogenously obtained.
Finally, they showcased the successful correction of multiple splicing defects in homozygous mutant photoreceptor precursor cells using a streamlined CRISPR/Cas9 editing strategy. This breakthrough not only confirmed minigene-transfected photoreceptor precursor cells as a more accessible cellular model for validation of splicing mutations but also identifies an efficient pathway for therapeutic applications.