How Mutant Proteins Impact Vision and Potential Therapeutic Strategies
Mutations in the rhodopsin gene (RHO) account for approximately 25% of all adRP cases. However, the potential mechanisms underlying how dominant mutations lead to photoreceptor cell death remain elusive.
Individuals with autosomal dominant retinitis pigmentosa (adRP) often experience symptoms such as night blindness, reduced peripheral vision, and, eventually central vision loss as the disease progresses. The loss of photoreceptor cells in the retina - rods, and cones - leads to the visual impairment observed in adRP cases. adRP is a genetic eye disease caused by a mutation that can be passed from an affected parent to their offspring. Mutations in the rhodopsin gene (RHO) account for approximately 25% of all adRP cases. However, the potential mechanisms underlying how dominant mutations lead to photoreceptor cell death remain elusive.
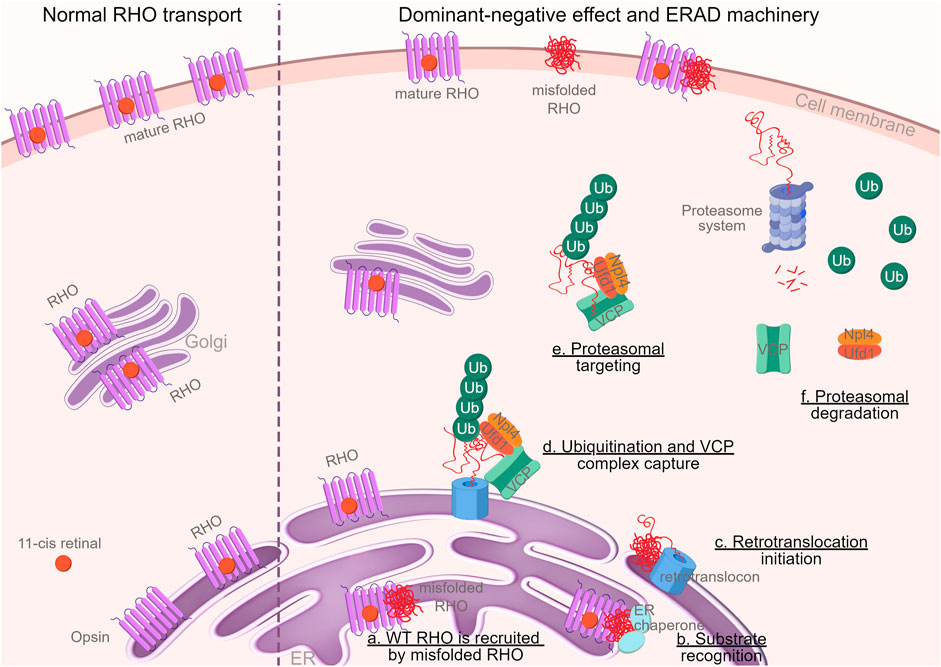
A study, conducted at the Institute for Ophthalmic Research and the Graduate Training Center of Neuroscience at the University of Tübingen, Germany, investigated the effect of the autosomal dominant mutation RHOΔI256. Using cell lines and retinal explants, the researchers found that mutant RHO protein forms aggregates and disrupts the normal trafficking of wild-type RHO within the cell cytoplasm, leading to cellular dysfunction. This disruption, known as the dominant-negative effect, emerged as a key player in the pathogenesis of adRP. In addition, the study highlighted that abnormal protein aggregation triggers endoplasmic reticulum (ER)-associated degradation system (ERAD). During this process, the ERAD effector protein, called valosin-containing protein (VCP), helps move the mutant RHOΔI256 aggregates from the endoplasmic reticulum (ER) to the cell's proteasome system, where these aggregates are broken down and degraded.
These results not only deepen our understanding of the underlying mechanisms of adRP but also pave the way for identifying potential therapeutic targets to restore proper protein trafficking and mitigate the detrimental effects of mutant RHO. In addition, these findings have the potential to lay the groundwork for the development of novel treatment strategies offering hope to individuals affected by adRP by aiming to slow or stop the progression of vision loss.
For further details, see publication in Front. Mol. Mutant dominant-negative rhodopsin∆I256 causes protein aggregates degraded via ERAD and prevents normal rhodopsin from proper membrane trafficking