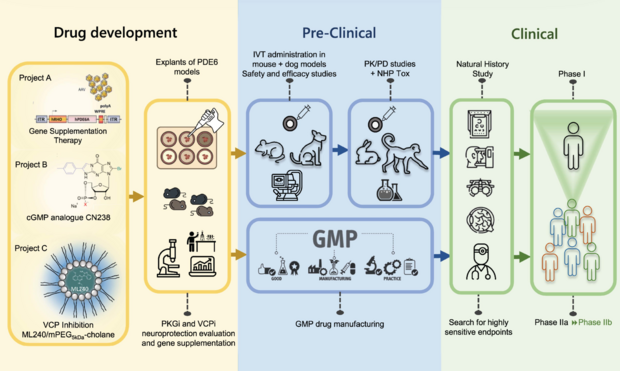
The R&D Plan
The RD TREAT project incorporates 3 independent mutually complementing approaches
The RD TREAT project aims at developing effective treatment for so far incurable inherited retinal dystrophies (IRD) with specific attention on visual loss due to dysfunction in the photoreceptor transduction cycle. Its Research and Development Plan delineates the steps and conditions required to obtain treatment within the next 5 years.
The project incorporates three independent mutually complementing approaches, a gene replacement regimen for the treatment of PDE6A-linked retinitis pigmentosa, and two mutation-independent pharmacological neuroprotective approaches.
Thanks to a strategic partnership with the Foundation for Medical Innovations (SfM) and the Faculty of Medicine, researchers from Institute for Ophthalmic Research will bring three promising compounds closer to clinical use.
This parternership marks the launch of RD TREAT (Retinal Dystrophies Treatment), a comprehensive program aimed at translating decades of research into real hope for patients.
The program focuses on 3 key projects:
- 1 Gene replacement therapy for specific types of retinitis pigmentosa (Project A)
- 2 pharmacological, mutation-independent therapies to protect retinal cells (Project B, Project C)
These cutting-edge strategies aim to halt disease progression and preserve vision for those affected by rare, currently incurable conditions.
With over 400 clinical staff members and 160 researchers, Tübingen’s Eye Clinic and Research Institute for Opthalmology form a powerhouse of innovation. The RD TREAT program strengthens their already recognized global leadership in ophthalmic care.
A dedicated translational team, formed with SfM, ensures smooth coordination from lab discoveries to clinical application.
The program is guided by:
- A 5-year strategic plan
- Streamlined decision-making processes
- Strong regulatory and project management support
Embedded within the “Gene and RNA Therapy Center (GRTC)”, RD TREAT serves as a pioneering model for personalized molecular therapies—potentially influencing treatments far beyond ophthalmology.
Besides the gene therapy, two pharmacological approaches have been developed, targeting Protein Kinase G (PKG) and Valosin-Containing Protein (VCP), respectively.
Both PKG and VCP inhibitors have shown mechanistic evidence to correct IRD-mutation-induced alterations in photoreceptor metabolism and thereby diminish retinal degeneration. Additionally, they have been demonstrated to preserve the viability of rod photoreceptors and improve the function of cones. Thus, PKG and/or VCP inhibitors bear the potential to preserve the functionality of human vision.
The central goal of the RD TREAT Plan is to provide clinical proof of concept for effective gene- therapeutic, as well as the pharmacological treatment options in the treatment of PDE6A-linked retinopathies and related disorders, within 5 years after the start of this project.
To reach this goal, RD TREAT roadmap outlines stringent plans for bringing three different approaches Projects A, B, C, that have already shown proof-of-principle in animal models, into the clinic.
Contact
For media or research inquiries:
Prof. Dr. Marius Ueffing
Director, Research Institute of Ophthalmology
Spokesperson, RD TREAT
+49 (0)7071 29-84020
+49 (0)7071 29-4560
marius.ueffing@uni-tuebingen.de